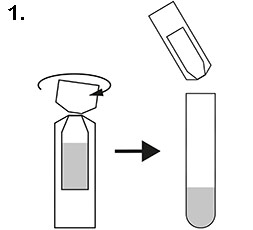
1. Open the test solution bottle and pour the solution into the test tube provided
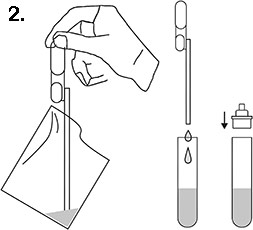
2. Use the pipette to take a sample of saliva and transfer this to the test tube containing the solution. Replace the cap on the test tube and shake to mix completely
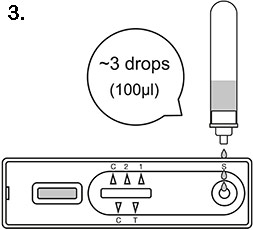
3. Carefully add 3 drops of the test sample into the test cassette well marked "S" and wait for 10 - 15 minutes. If left unread for 20 minutes or more the results are invalid
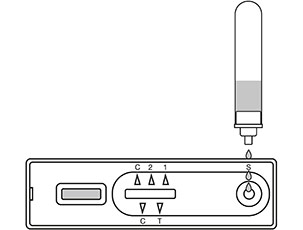
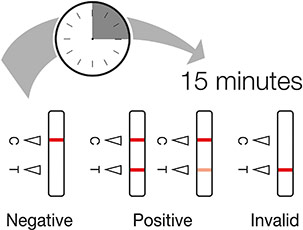
Once 10-15 minutes has passed check the results indicator
The "C" (control) line must have a red mark or the test is invalid
The "T" (test) line is red for a positive result (even a faint line is a positive result)
The Panodyne COVID-19 Antigen Rapid Lateral Flow Test Kit (saliva sample), which has gained CE certification, is intended for Health Professional Use in various settings to help identify whether, for example, visitors to those in care homes (at the time of testing) have COVID-19 (Coronavirus) – even if no obvious symptoms are present.
We recommend you read the Official Government Guidance regarding SARS-COV-2 Lateral Flow Tests here.
It’s quick and accurate. The point of care test uses a saliva sample to detect the presence of the antigen produced by the body’s immune system, and a positive or negative result is visible within 15 minutes of testing, with an accuracy rate of up to 93.33%.
Most tests require both a nose and throat swab in order to work correctly, however this test has been specifically formulated to work with saliva so there's no discomfort. It's also easier to use for vulnerable people or those with a disability.
These tests can be administered to both Adults & Children.
Each individual test kit includes 1x test cassette, 1x test solution, 1x test tube, 1x sample collection bag, and 1x pipette dropper, which are all located inside a sealed test kit wallet.
The test kit works very quickly, requires no specialist equipment, is easy to use, and test results are easy to read. It can be self-administered under supervision by trained staff, a healthcare professional, company nurse, or occupational health personnel as they do not require any medical training. Also, please note that if you are experiencing any COVID-19 symptoms, you must follow Government guidelines in terms of isolating and testing.
Please note that this test is not intended for home use.
HS Code: 3822001000
A recent article was published on the National Health Executive website about Panodyne tests which can be read here.
Panodyne have produced a very useful Declaration Form for each of their test kits as attached which can be used each time someone is tested and kept as an internal record. Unlike PCR test kits, Lateral flow test kits do not need to be sent off to a lab to be processed and will provide results within 10-15 minutes. If the individual tested is positive, it is recommended that they immediately self-isolate and arrange to take a PCR test.
Panodyne sets the industry standards for unrivalled customer care and excellent client services from order to delivery, providing prompt replies and swift resolution to any queries. All Panodyne products are fully accredited and CE certified and registered with the MHRA where applicable.
With over 20 years of expertise, Panodyne is a well-established and trusted UK manufacturer with full ISO9001:2015 and ISO:13845 quality management system accreditations. Its production facilities are regulated and audited under the strictest standards to guarantee high quality consistency and product availability, while products are also independently tested by global testing agencies such as SGS and Intertek. All products and medical devices are researched, designed and formulated in the UK and tested to the highest European standards to meet all legal requirements.
Hover over the icon (if applicable) for more information
Specifications are subject to change - See Terms & Conditions for more info